Last Updated on March 24, 2023 by Francis
Attractive forces are the forces that hold particles together to form molecules and compounds. Although some of these forces, such as covalent bonds, are very strong, there is one type of attractive force that is much weaker. In this article, we’ll discuss what the weakest attractive force is and why it is weaker than the other forces.
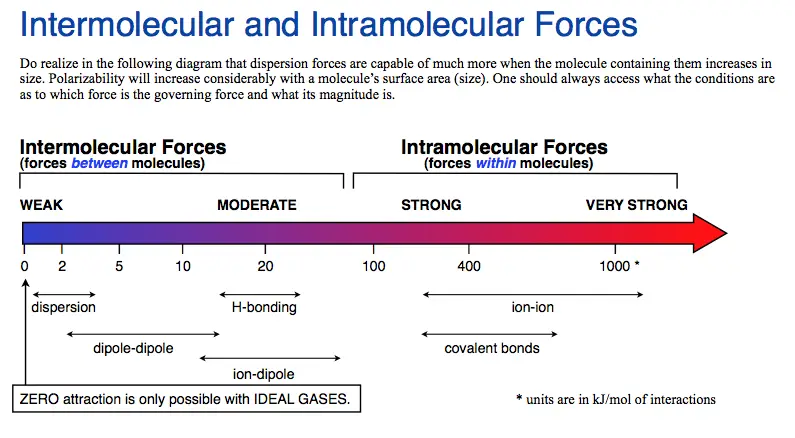
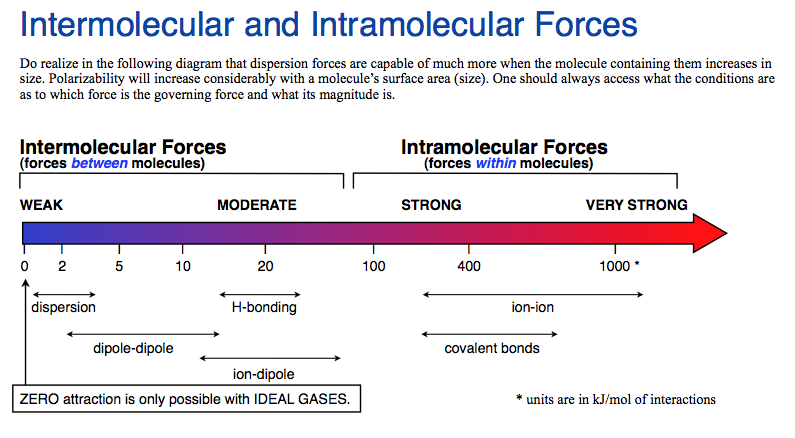
The weakest attractive force is the Van der Waals force. It is a weak intermolecular force that binds atoms and molecules together. It is much weaker than the other three intermolecular forces: hydrogen bonding, dipole-dipole interaction, and ion-dipole interaction.
Contents
Weakest Attractive Force: Van der Waals Force
Van der Waals force is the weakest attractive force in nature and is responsible for the cohesion of molecules. It is a non-specific interaction between atoms, molecules, and other particles that exists due to the electrostatic attraction between their permanent or induced dipoles. It is a relatively weak intermolecular force compared to stronger forces such as ionic, covalent, and metallic bonding.
Van der Waals force is a result of the fluctuating electromagnetic field that is created by the movement of electrons in atoms and molecules. This fluctuating field causes the electrons to be attracted to one another, leading to the formation of a weak bond known as a Van der Waals bond. This type of bond is weaker than other types of bonds, such as covalent bonds, and can be broken easily.
Van der Waals force is the main force responsible for the binding of the molecules of gases and liquids. It is also responsible for the formation of weakly bound complexes between molecules of different species. This type of force is important for the cohesion of biological molecules and for the stability of proteins and other macromolecules.
Van der Waals Force and Intermolecular Interactions
Van der Waals force affects the intermolecular interactions between molecules. It is a non-specific, weak attraction between molecules that arises due to the electrostatic attraction between their permanent or induced dipoles. This force is responsible for the cohesion of molecules, allowing them to form liquid or solid states.
Van der Waals force is responsible for the formation of weakly bound complexes between molecules of different species. This force is important for the formation of hydrogen bonds and other weakly bound complexes between molecules, such as those found in proteins and other macromolecules.
Van der Waals force is also responsible for the formation of weakly bound ion pairs, which are important in the formation of ionic bonds and the solubility of compounds in aqueous solutions. This type of force is also responsible for the formation of van der Waals complexes, which are important in the formation of polymers.
Van der Waals Force vs. Other Forces
Van der Waals force is weaker than other forces such as covalent and ionic bonds. It is also weaker than hydrogen bonding and other weakly bound complexes. This type of force is responsible for the cohesion of molecules, allowing them to form liquids and solids.
Van der Waals force is also weaker than the attractive forces between molecules. This type of force is responsible for the formation of weakly bound complexes between molecules of different species. This type of force is important for the cohesion of biological molecules and for the stability of proteins and other macromolecules.
Van der Waals force is also weaker than the forces between atoms. This type of force is responsible for the formation of van der Waals complexes, which are important in the formation of polymers. It is also responsible for the formation of weakly bound ion pairs, which are important in the formation of ionic bonds and the solubility of compounds in aqueous solutions.
Examples of Van der Waals Force
Van der Waals force is responsible for the cohesion of molecules, allowing them to form liquids and solids. Examples of this type of force include the attraction between molecules of different species that leads to the formation of weakly bound complexes, such as hydrogen bonds and other weakly bound complexes between molecules.
Van der Waals force is also responsible for the formation of van der Waals complexes, which are important in the formation of polymers. This type of force is also responsible for the formation of weakly bound ion pairs, which are important in the formation of ionic bonds and the solubility of compounds in aqueous solutions.
Van der Waals force is also responsible for the cohesion of biological molecules and for the stability of proteins and other macromolecules. This type of force is also responsible for the formation of weakly bound complexes between molecules of different species. These types of interactions are important for the functioning of biological systems.
Frequently Asked Questions
What is the weakest attractive force?
The weakest attractive force is the Van der Waals force. This force is a result of the electrostatic attraction between particles that are close to each other. It is much weaker than other forces such as ionic, covalent, or metallic bonding.
Van der Waals forces are attractive forces between atoms or molecules that are caused by temporary dipole-dipole interactions. These forces are attractive between all molecules, regardless of their polarity. They are responsible for holding together many different types of molecules, including water and carbon dioxide.
What causes Van der Waals forces?
Van der Waals forces are caused by temporary dipole-dipole interactions. This means that the electrons in one atom or molecule can temporarily move to create a temporary dipole, which can then be attracted to the dipole of another atom or molecule. This creates an attractive force between the two particles.
How are Van der Waals forces different from other attractive forces?
Van der Waals forces are different from other attractive forces in that they are much weaker than other forces such as ionic, covalent, or metallic bonding. They are also attractive between all molecules, regardless of their polarity. Additionally, Van der Waals forces are only effective at short range and decrease in strength as the distance between the two molecules increases.
What is the range of Van der Waals forces?
The range of Van der Waals forces is relatively short, typically only a few nanometers. As the distance between the two particles increases, the strength of the force decreases. This means that Van der Waals forces are only effective at short range and become weaker as the distance between the two molecules increases.
What types of molecules are held together by Van der Waals forces?
Van der Waals forces are responsible for holding together many different types of molecules, including water and carbon dioxide. They are also responsible for the attraction between non-polar molecules, such as hydrocarbons. Additionally, Van der Waals forces are important for the formation of weak hydrogen bonds, which are responsible for the structure of proteins and DNA.
What are some applications of Van der Waals forces?
Van der Waals forces are important in many different fields. They are important for the formation of weak hydrogen bonds, which are responsible for the structure of proteins and DNA. Additionally, Van der Waals forces are important in materials science and are used to measure the surface tension of liquids. They are also important for the formation of colloids, which are used in many industrial and medical applications.
The Weakest Attractive Force
The weakest attractive force is a difficult concept to quantify, as there are so many different forces that can attract one object to another. However, when it comes to physics, the weakest attractive force is the van der Waals force, which is a result of the fleeting dipole–dipole interactions between molecules. This force is relatively weak, but it can still have an impact on certain processes, such as why liquids and solids take their shape. Ultimately, it is the van der Waals force that is the weakest attractive force in the physical world.